Inhibitors - Info
Inhibitors are molecules that compete with substrates for the active binding sites of enzymes. In general there are two main inhibition principles - irreversible and reversible inhibition. In the first case the inhibitor binds irrevocably to the active site of the enzyme which results in a loss of function. While in the second case there are several mechanisms which will be explained in more detail in the following section. Despite all negative characteristics inhibitors are associated with they play a decisive role in metabolic regulations of organisms.
How do inhibitors work?
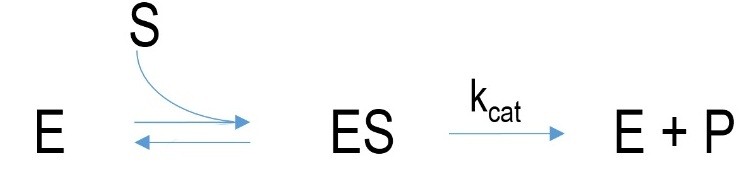
Figure 1 Schematic mechanism of an enzyme-mediated reaction. E= enzyme, ES=enzyme-substrate-complex, P=product, k=velocity constant
Irreversible inhibition
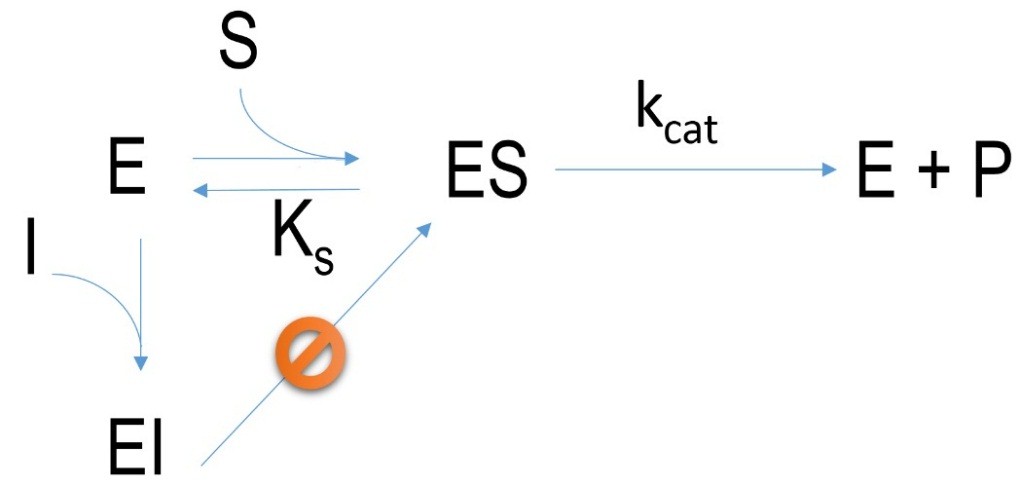
Figure 2 Schematic mechanism of an irreversible inhibition. E=enzyme, I= inhibitor, EI=enzyme-inhibitor-complex, ES=enzyme-substrate-complex, P=product, k=velocity constant, K= dissociation constant
Inhibitors which bind covalently at an active site of an enzyme are irreversible. This kind of inhibition cannot be changed. Many of such inhibitors are called “suicide inhibitors” because they promote drastic physiological reactions in organisms which can lead to death. Some examples are: sarine gas, pesticides: DDT, parathion, antibiotics: penicillin.
Reversible inhibition
Competitive
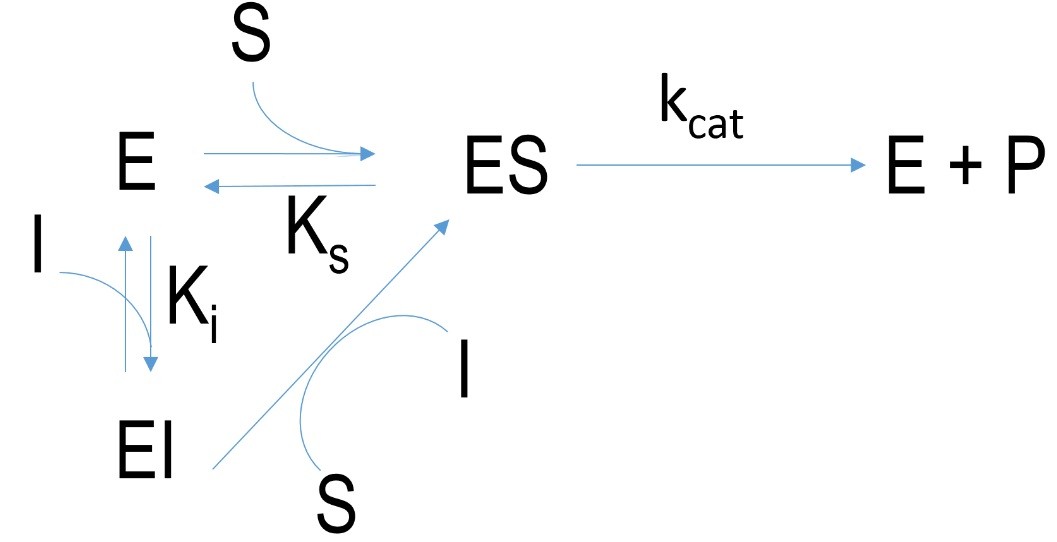
Figure 3 Schematic mechanism of a competitive inhibition. E=enzyme, I= inhibitor, EI=enzyme-inhibitor-complex, ES=enzyme-substrate-complex, P=product, k=velocity constant, K= dissociation constant
Competitive inhibition describes inhibitors which bind at the active site of enzymes but not covalently. The interaction between enzyme and inhibitor is weaker (H-bonds, ionic bonds) so the substrate and inhibitor can compete over the active binding site. By increasing the concentration of substrate the bindings of inhibitors can be overcome in contrast to irreversible inhibition.
Allosteric or non-competitive
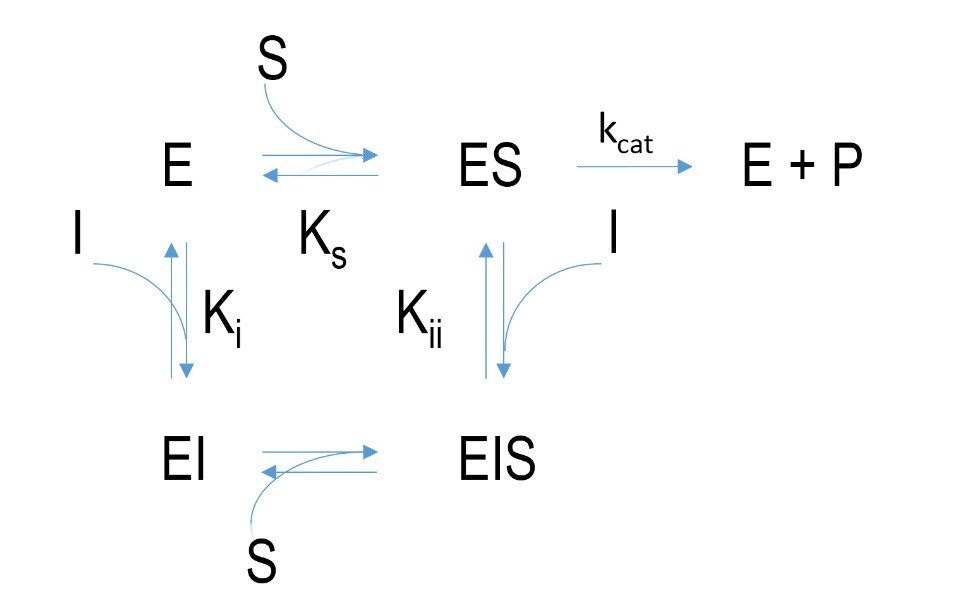
Figure 4 Schematic mechanism of a non-competitive inhibition. E=enzyme, I= inhibitor, EI=enzyme-inhibitor-complex, ES=enzyme-substrate-complex, EIS= enzyme-inhibitor-substrate-complex, P=product, k=velocity constant, K= dissociation constant
Allosteric or non-competitive inhibition is different compared to competitive inhibition. The inhibitor does not bind at the active site but at another location of the enzyme. An enzyme-inhibitor-complex forms. This leads to a change in protein conformation of the enzyme which can affect the conversion of substrate to product. The binding of an inhibitor in contrast to irreversible or competitive inhibition does not prevent substrate binding. Besides binding to non-bound enzyme an inhibitor can bind to the already formed enzyme-substrate-complex which results in an enzyme-inhibitor-substrate-complex. This leads to a delay in releasing of the product at the active site. Increasing the substrate concentration does not enhance binding of the substrate though.
Feedback inhibition
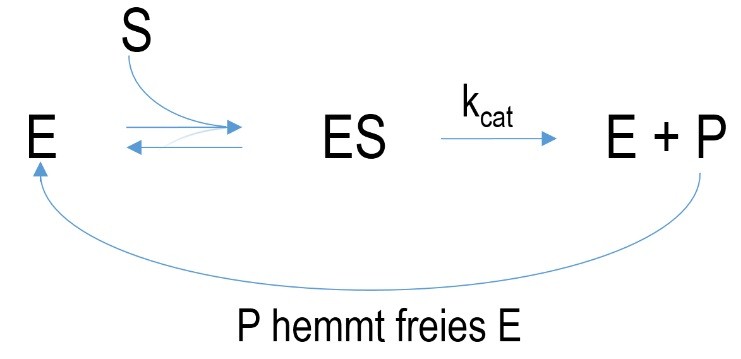
Figure 5 Schematic mechanism of a feedback inhibition. E= enzyme, ES=enzyme-substrate-complex, P=product, k=velocity constant, K= dissociation constant
Feedback inhibition is a special case because the generated product of an enzyme-catalyzed reaction is the inhibitor of its enzyme. Hence an accurate regulation of substrate conversion is possible which denies overproduction of product.
Application
Inhibitors especially such that bind irreversibly are of great use for pharma companies because they are able to inhibit metabolic enzymes very specifically. Another advantage is the non-reactiveness outside of its application area – the active site. Some examples are:
DFMO (difluoromethylornithine): inhibits ornithine decarboxylase of Trypanosoma which cause sleeping sickness.
DFP (diisopropylfluorophosphate): inhibits acetylcholine esterase, which catalyzes the reaction of acetylcholine and water to choline and acetate.
Penicillin: inhibits glycopeptidetranspeptidase of bacteria which is responsible of cell membrane synthesis. (1)
There are several other sectors inhibitors are of essential importance, such as: cancer research, mechanisms of bacterial/viral infections or general research of enzyme-catalyzed reactions.
Convince yourself of our inhibitor experts!
Literature
- Stryer et al. Biochemisty 5th edition, ch.8.5, p. 330-335
- Campbell and Reece, Biology 8th Edition, ch. 8.4, p. 156
- http://www.chemgapedia.de/vsengine/vlu/vsc/de/ch/8/bc/vlu/biokatalyse_enzyme/enzymhemmung.vlu/Page/vsc/de/ch/8/bc/enzymregulation/suicide_inh.vscml.html status: 04.04.2016 15:30



