What are Antibodies?
Products
Definition of antibodies
Antibodies are a class of proteins that play important roles for the immune system. They are made by B-lymphocytes (a type of white blood cells) and by plasma cells in response to substances which are foreign for the organism (antigens = antibody generating). Antibodies are also called immunoglobulins and make up the main part of the specific endogenous defence.
Today, antibodies are used in medicine as well as in biology for diverse diagnostic assays like drug tests, ELISA, ELISPOT, EMSA, FACS, pregnancy tests and western blot.
How is an antibody structured?
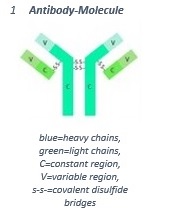
Both, the heavy and the light chains, contain a constant (C-region) and a variable (V-region) region. While the sequence of amino acids within the C-region varies only a little between the different classes of antibodies, the sequence of the V-region at the end of the Y-arms is highly variable.
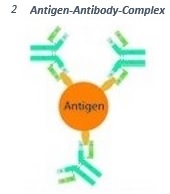 Antibodies are able to detect and bind small molecular structures at the surface of antigens (epitopes). This way the so called antigen-antibody-complex is formed (2).
Antibodies are able to detect and bind small molecular structures at the surface of antigens (epitopes). This way the so called antigen-antibody-complex is formed (2).
The V-regions determine the specific binding sites of the antibody. These are able to detect and bind the respective antibody. Every antibody contains at least two binding sites directed against the antigen which has initiated its production.
How do antibodies function?
Most of the antigens are proteins (for example of bacteria or viruses), but also lipids, carbon hydrates and other substances can exhibit antigenic effects. The goal of binding antigens via antibodies is to inactivate and eliminate them. There are various mechanisms for this:
Blocking a specific site of an antigen which is necessary to exert its effects (e.g. detection and entering a cell of the organism) via antibodies is called neutralisation.
The attachment of antibodies to the locomotion system of intruders (for example the flagella of bacteria) leads to an immobilisation.
Some foreign substances need to be marked as such by the organism in order to get recognized by defence cells and phagocytosed (“eaten”) subsequently. This marking can be exerted among others by antibodies and is called opsonisation.
Agglutination and precipitation are two processes which lead to linking or clumping of antigens via antibodies. Larger antigen-antibody-complexes are easier to detect and digest by phagocytes.
The complement system can be activated by antigen-antibody-complexes. The complement system consists of about 25 different proteins which are able to support the elimination of antigens by enhancing reactions (e.g. opsonisation, inflammatory reactions) related to immune responses.
Which antibody classes exist?
Antibodies are divided into different classes according to the differences within the sequence of the C-region. In mammals exist five classes of immunoglobulins, defined by five differently structured C-regions:
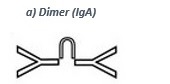 • IgA (dimer, see a) takes function as a first barrier against intruders, because of its presence in mucous membranes (mouth, nose, eyes and intestinal tract). Many foreign substances are already intercepted and eliminated by IgA. Besides mucous membranes IgA is also secreted in glands near to the maternal acromastium and transported into infants via the mother´s milk.
• IgA (dimer, see a) takes function as a first barrier against intruders, because of its presence in mucous membranes (mouth, nose, eyes and intestinal tract). Many foreign substances are already intercepted and eliminated by IgA. Besides mucous membranes IgA is also secreted in glands near to the maternal acromastium and transported into infants via the mother´s milk.
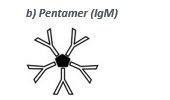 • IgM (pentamer, see b) is the first antibody produced after an infection (primary response). After a few weeks the IgM titer decreases and is replaced by IgG, which is produced in the meantime.
• IgM (pentamer, see b) is the first antibody produced after an infection (primary response). After a few weeks the IgM titer decreases and is replaced by IgG, which is produced in the meantime.
 • IgG (monomer, see c) is the most frequent antibody. It circulates within body secretions like blood and lymph and is part of a delayed immune response (secondary response). The production of IgG starts three weeks after an infection, but it remains in the body for a long time. Based on an IgG titer past infections and vaccinations can be detected.
• IgG (monomer, see c) is the most frequent antibody. It circulates within body secretions like blood and lymph and is part of a delayed immune response (secondary response). The production of IgG starts three weeks after an infection, but it remains in the body for a long time. Based on an IgG titer past infections and vaccinations can be detected.
- • IgD (monomer, see 3c) occurs only in low concentrations in blood and lymph. At the surface of B-lymphocytes it operates as antigen receptor and supports their reproduction and differentiation. Thus, IgD supports the production of antibodies during an infection.
- • IgE (monomer, see 3c) is just a minor part of the whole antibody pool. In its free form it is nearly not present within blood and lymph. The IgE molecule is predominantly found at the surface of mast cells (cells of the immune system, which store messenger substances like histamine and heparin) and basophils (a form of white blood cells) where it stimulates the distribution of histamines and other inflammatory supporting substances in case of contact with antigens. This leads to an expansion of blood vessels which enables much easier the movement of other immune cells to the place of infection.



