Protein modifications – acetylation, ubiquitinylation, phosphorylation, SUMOylation
Protein modifications – acetylation, ubiquitinylation, phosphorylation, SUMOylation
Definition: Modification
In a covalent bond, the reacting atoms share an electron pair ("valence electrons") and thus form a chemical bond. This process is usually catalysed by enzymes. The result is a covalent bond or modification
The covalent attachment of another molecule can modify the activity of an enzyme or other proteins. In these cases, a donor molecule provides a functional part that changes the properties of the enzyme. Most modifications are reversible. This is also referred to as reversible covalent modification.... Phosphorylation and dephosphorylation are the most common covalent modifications in biological systems. However, there are also others, e.g. acetylation, ubiquitinylation and SUMOylation.
Types of modification
Acetylation
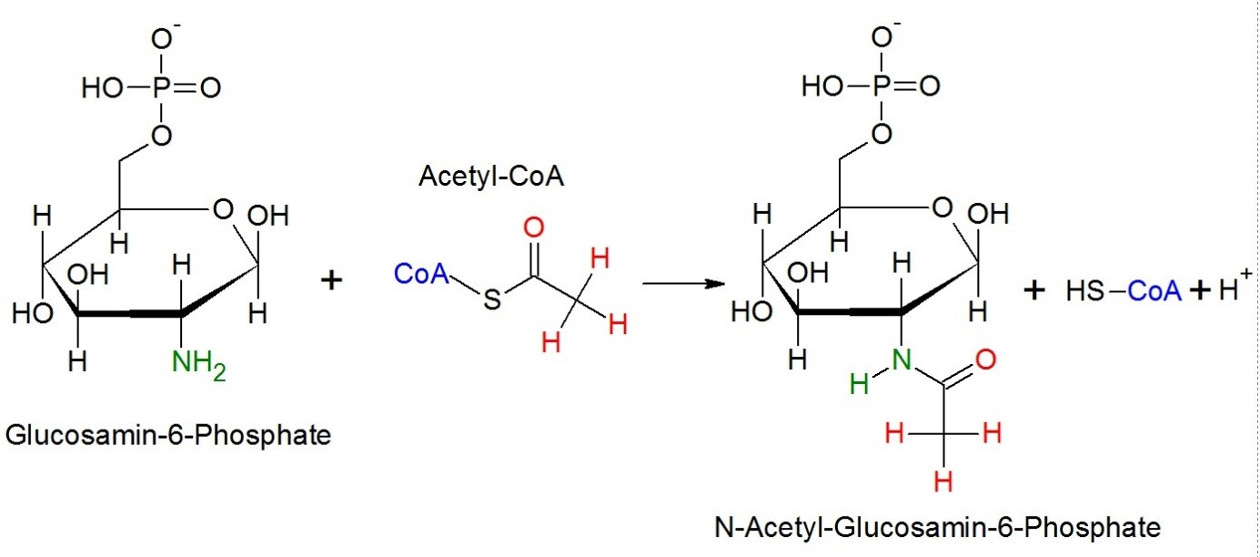
Figure 1: Acetylatio; Example – Glucosamin-6-phosphate. CoA is a acetyltransferase and transfers its acetylrest onto the aminogroup of glucosamin-6-phosphate.
Acetylation and deacetylation play an important role in transcription of genes. Concretely, acetylated histones are connected to actively transcribed genes, whereas methylated histones show inactive or low transcribed gene areas. Acetylated and methylated gene areas serve as recognition sequences for RNA and DNA polymerases.
The acetylation and deacetylation of proteins thus regulates their function.
This is a post-translational protein modification. An acetylase transfers an acetyl group from acetyl-coenzyme A to the target protein, usually to a lysine.
The deacytlase can remove the acetyl group again and thus makes the protein acetylation reversible.
Phosphorylation
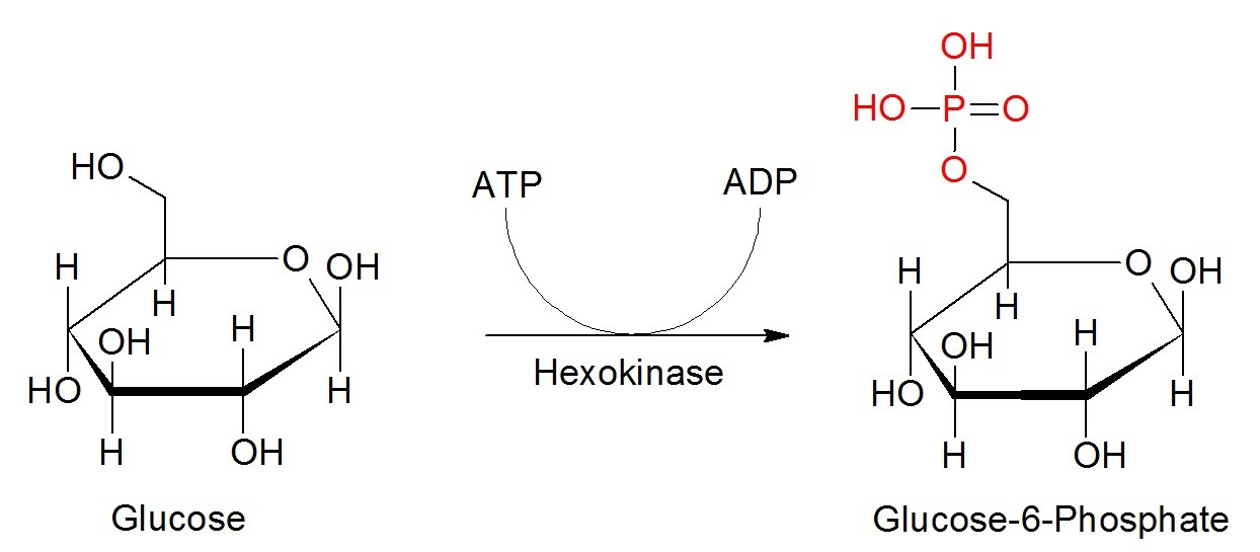
Figure 2: Phosphorylation; Example – Glucose. Glucose is phosphorylated by the help of hydrolysis of ATP to ADP and the transfer of the phosphate onto its C6-atom by hexokinase
In general, phosphorylation is needed for activation and regulation of molecules, especially enzymes.
A phosphate group is attached to an organic molecule by a phosphate kinase.
In the case of proteins, this creates phosphoproteins, which play a central role in our nervous system. Well-known examples that are regulated by phosphorylation are enzymes (e.g. receptor tyrosine kinases, MAP kinases) and transcription factors (e.g. c-Jun, CREB).
In the case of proteins, this creates phosphoproteins that play a central role in our nervous system.
Ubiquitinylation
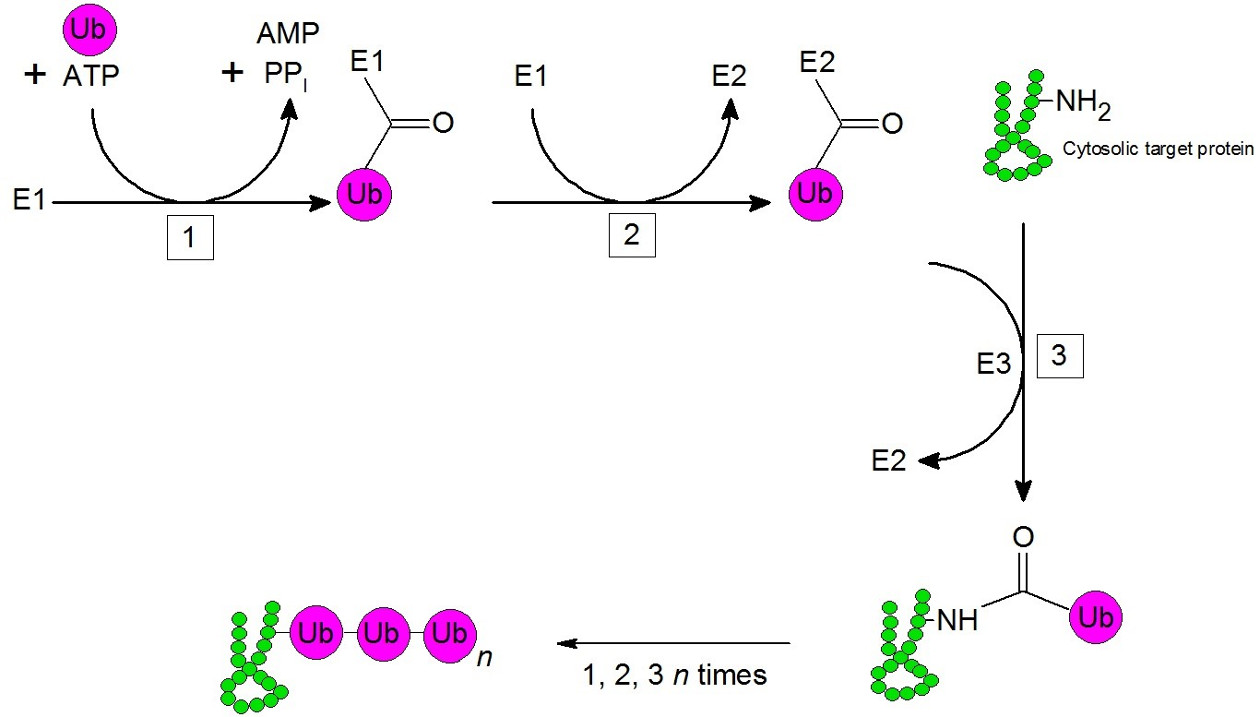
Figure 3: Ubiquitinylation; Example – cytosolic protein. 1.) Enzyme E1 activates in an ATP-dependent step ubiquitin and subsequently, ubiquitin will be transferred onto E2. An ubiquitin ligase (E3) transfers the bound ubiquitin onto an amino residue of a lysin of the target protein. Additional ubiquitin molecules are being added onto the target protein by repeating of step 1-3. The polyubiquitinylated protein is now marked for degradation at the proteasome.
When ubiquitin is transferred to a target protein, this is called ubiquitination. This results in the protein being degraded in the proteasome.
A further differentiation is made between mono- (single ubiquitin molecule) and polyubiquitination (ubiquitin chains) of proteins.
SUMOylation
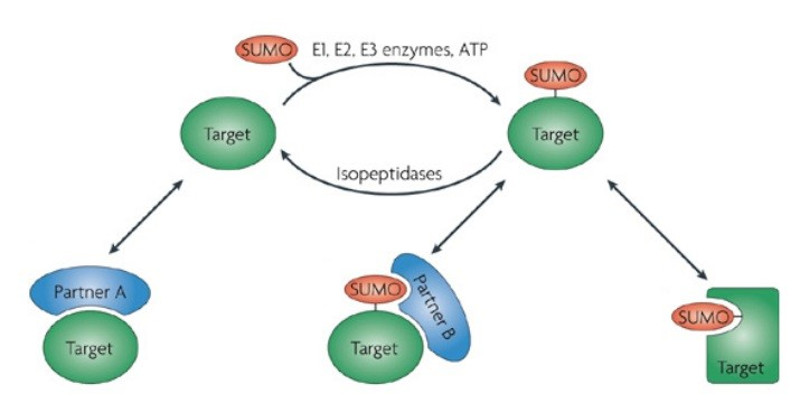
Figure 4: SUMOylation
The covalent attachment of SUMO proteins (Small Ubiquitin-related MOdifier) to the lysine residues of proteins is called SUMOylation. The reverse of this covalent modification is called "DeSUMOylation".
Offers of Hölzel Diagnostika
Signal Seeker™ Kits - tools for research on signalling pathways:
Signal Seeker™ Kits aim to enable scientists to discover new signalling pathways, which include dynamic protein modifications such as ubiquitylation, SUMOylation or phosphorylation. The strength of Signal Seeker™ Kits lies in their optimized reagents, which allow the detection of protein modifications within a nanogram scale. The BLASTR™ Buffer System allows the user to examine varying changes in protein modifications. Signal-Seeker™ Kits further offer a customer-friendly introduction into the usually quite complex world of mechanisms behind the dynamic signalling pathways. The detailed Kit manuals provide a good amount of information for the user. Certain Kit reagents that exhibit affinity beads and antibodies are also separately available. Especially the ubiquitin affinity beads are substantially better than commonly used affinity beads, due to their affinity for ubiquitinated proteins, including those modified by the ubiquitin monomer. The literature cites two references of a formerly developed product.
Various products:
- Signal Seeker™ Phosphotyrosine Enrichment Kit 30
- Signal Seeker™ Ubiquitin Enrichment Kit 30
- Signal Seeker™ Sumoylation Enrichment Kit 30
Literature
- Stryer et al. Biochemistry 5th Edition, Ch. 10.4, p. 423
- Anti-phosphorylation affintiy beads (Cat. # BK160), Law A. et al. 2015. Temporal regulation of phosphotyrosine-modified Rac1 in response to epidermal growth factor (EGF) stimulation. American Society for Cell Biology, 2015, Poster P2126.
- Anti-acetyl-lysine monoclonal antibody (Cat. # AAC01), LaBarge et al. 2015. p300 is not required for metabolic adaptation to endurance exercise training. The FASEB Journal article doi: 10.1096/fj.15-281741. This paper uses anti-acetyl lysine to probe extracts of transgenic mouse muscle tissue, looking for the effects of a knocked out HDAC gene.



