Proteins - Info
What are Proteins?
Products
Definition of proteins
Proteins are macromolecules with various forms and functions within the organism. Among others they play an important role in:
- • metabolic processes (enzymes)
- • signal transduction within the organism (hormones, receptors, membrane transporters)
- • cell growth and differentiation (cytokines)
- • transport of substances (haemoglobin, albumin, transferrin, thyroxin binding globulin)
- • stabilisation of supporting structures (collagen, elastin)
- • defence against pathogens (antibodies)
Just as manifold the functions of proteins are their forms. Proteins are made of a kit of twenty different amino acids, which are assembled into long chains (polypeptides) via covalent bonds. The amino acid sequence of polypeptides is encoded by DNA in the cell nucleus. A modified copy of a defined part of the DNA (gene) is generated by the enzyme RNA-Polymerase (transcription). After additional modifications a complete construction plan for a protein (messenger RNA) is exported out of the cell nucleus. Within the cytosol the construction plan is read by an enzyme complex (ribosome), and the respective amino acids are bound to each other in the correct order (translation). A polypeptide larger than 100 amino acids is called protein, shorter chains are called peptides.
Structure of proteins
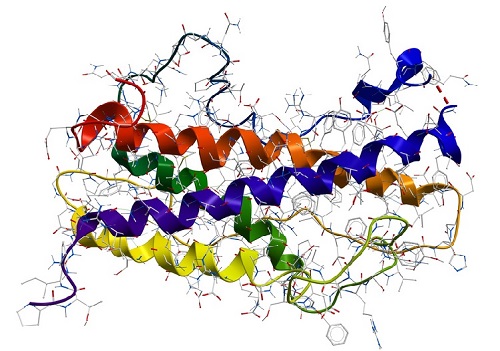
Proteins can be made of one or more polypeptides. The amino acid sequence of polypeptides is also named the primary structure of a protein. The conformation of a protein is defined by hydrogen bonds which can occur in regular distances along the polypeptide backbone. Every protein contains two repeating structures, resulting from hydrogen bridges: α-helix and β-sheet. Those are also referred to as the secondary structure of a protein. The spatial arrangement of α-helices and β-sheets is caused by hydrophobic interactions (van der Waals forces) and also called the tertiary structure of a protein. This can be stabilised additionally by disulfide bridges between two amino acids cysteine. If a protein consists of two or more polypeptides the aggregation of those to a functional molecule is the so-called quaternary structure of a protein.
Protein conformation
A protein conformation refers to the three-dimensional shape or arrangement of a protein molecule in space. Proteins are complex biomolecules made up of chains of amino acids that fold into specific three-dimensional structures, which are critical to their biological function. The sequence of amino acids in a protein determines its primary structure, and the way in which the protein folds into a specific shape is called its conformation or tertiary structure.
Protein conformation is determined by various factors, including the sequence and properties of the amino acids, as well as the interactions between different parts of the protein molecule, such as hydrogen bonding, electrostatic interactions, van der Waals forces, and hydrophobic interactions. These interactions determine how the protein folds and organizes itself in space to adopt a stable three-dimensional structure.
Proteins can have different types of conformations, including alpha helices, beta sheets, loops, turns, and other structural motifs. The specific conformation of a protein is critical to its function, as it determines how the protein interacts with other molecules in its environment, such as other proteins, nucleic acids, or small molecules. Changes in protein conformation can have significant effects on protein activity, stability, and binding properties, and can be triggered by various factors, such as changes in pH, temperature, or the presence of specific molecules.
Understanding protein conformation is essential in fields such as biochemistry, molecular biology, and drug discovery, as it provides insights into protein structure-function relationships, protein-protein interactions, and drug-protein interactions. Experimental techniques, such as X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, and computational modeling, are used to study protein conformation and elucidate the complex three-dimensional structures of proteins.
Denaturation of proteins
A decisive role for the specific function of proteins is played by the conformation. So a loss of conformation (denaturation) does mostly mean also loss of activity. Several external conditions like high temperature, high-energy radiation or various chemicals such as salt, acids and bases may have a negative influence on form and function of proteins.
A denaturation is able to break hydrogen bonds, disulfide bridges, hydrophobic interactions and ionic bonds, but not the covalent bonds between amino acids. For this reason only the secondary and tertiary structure (and also the quaternary structure, if existing) of proteins are affected by structural changes during denaturation, whereas the primary structure will be preserved.